NextGene™ SARS-CoV-2 Fast Detection Kit
- Shortening PCR amplification time less than 1 hour
Clinical sensitivity 100% and clinical specificity 99.54% based on the clinical performance evaluation
Avoiding the risk of contamination by applying UDG
Monitoring whole PCR process by endogenous IC (Internal Control)
Product Summary
MFDS approval
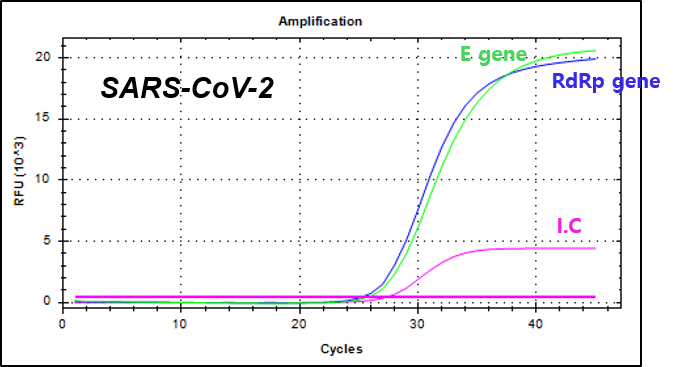
NextGene™ SARS-CoV-2 Fast Detection Kit is a real-time RT-PCR based in vitro diagnostic medical device designed to qualitatively detect both E gene and RdRp gene of the novel coronavirus (SARS-CoV-2) from human nasopharyngeal or oropharyngeal swab samples.
DETAIL
-
Detection Target
E gene and RdRp gene of SARS-CoV-2
-
Specimen
Nasopharyngeal swab, Oropharyngeal swab
-
Limit of Detection
12.25 copies/µL
-
Component
2X qRT-PCR Fast Enzyme Mix
Oligo Mix (SARS-CoV-2)
Positive Control (SARS-CoV-2)
Negative Control -
Validated Instrument
CFX96 Dx system
-
Order No.
RV1001A (50 rxns / kit)
RV1001B (100 rxns / kit)
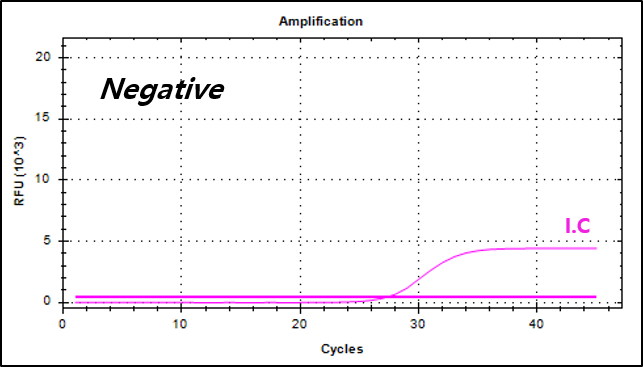
Example of Result