NextGene™ STI-12 Detection Kit 5/CGE (RUO)
- Simultaneous multiplex detection of three (3) types of sexually transmitted diseases in a single tube reaction
Avoiding the risk of contamination by applying UDG
Validation of PCR amplification by applying PCR Control
제품소개
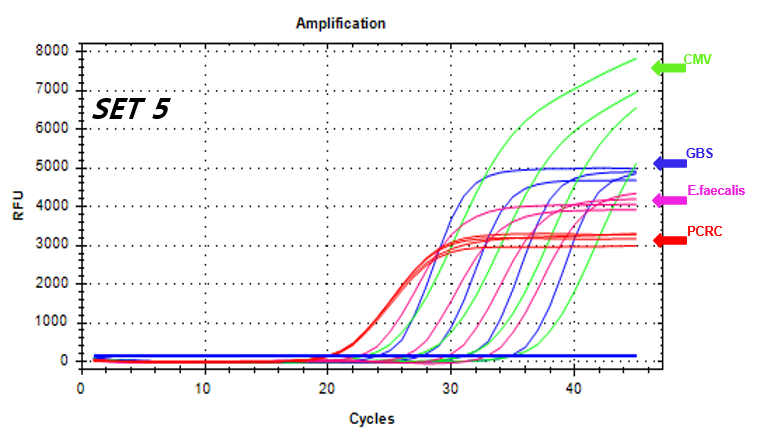
NextGene™ STI-12 Detection Kit 5/CGE is a real-time PCR based reagent for research use, designed to simultaneously and qualitatively detect Cytomegalovirus, Streptococcus agalactiae (group B streptococcus), and Enterococcus faecalis, which are causes of perinatal infections, from human vaginal swabs or urine samples.
제품정보
-
Detection Target
Cytomegalovirus
Group B streptococcus
Enterococcus faecalis -
Specimen
Vaginal swab, Urine
-
Limit of Detection
10 copies/reaction
-
Component
2X qPCR Enzyme Mix
Oligo Mix (STI 5/CGE)
Positive Control (STI 5/CGE)
Negative Control -
Validated Instrument
CFX96 Dx system
-
Order No.
EQD97-K050 (50 rxns / kit)
EQD97-K100 (100 rxns / kit)
결과 그래프 (예시)