NextGene™ MTB/NTM Detection Kit
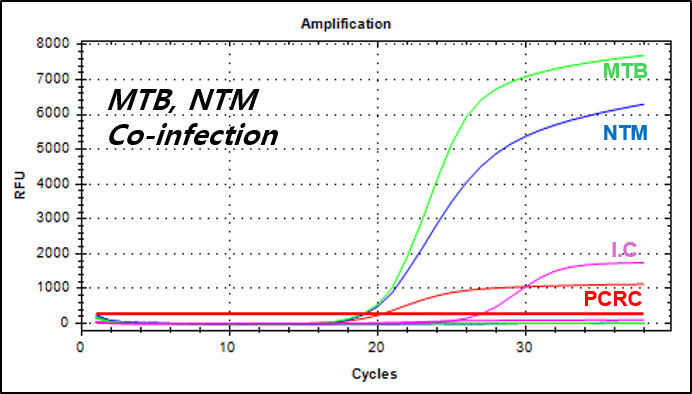
- Simultaneous detection of Mycobacterium tuberculosis and Nontuberculosis mycobacteria
Avoiding the risk of contamination by applying UDG
Monitoring whole PCR process by endogenous IC (Internal Control) and PCR Control as well
제품소개
MFDS approval
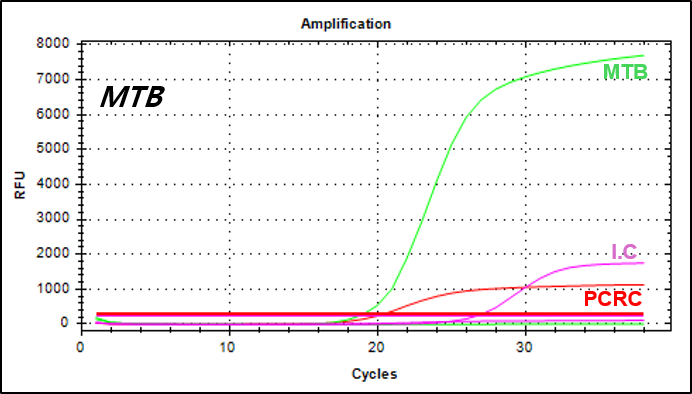
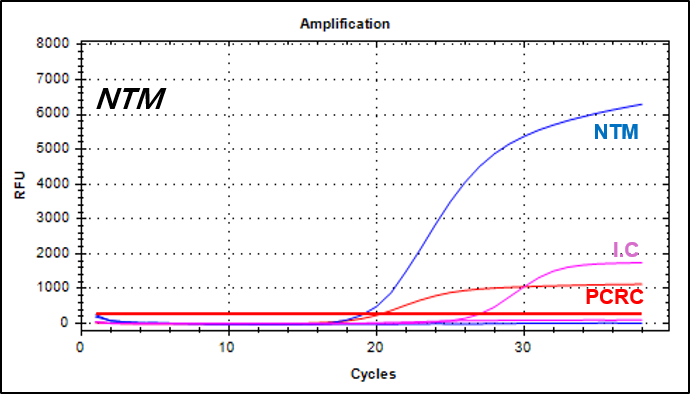
NextGene™ MTB/NTM Detection Kit is a real-time PCR based in vitro diagnostic medical device designed to simultaneously and qualitatively detect Mycobacterium tuberculosis and nontuberculosis mycobacteria from human sputum or cultured cell samples.
제품정보
-
Detection Target
Mycobacterium tuberculosis
Nontuberculosis mycobacteria -
Specimen
Sputum, Cultured cell
-
Limit of Detection
Mycobacterium tuberculosis (MTB) : 1.3 copies/µL
Mycobacterium avium (NTM) : 1.32 copies/µL
Mycobacterium intracellulare (NTM) : 7.4 copies/µL
Mycobacterium abscessus (NTM) : 3.24 copies/µL
Mycobacterium fortuitum (NTM) : 0.86 copies/µL
Mycobacterium kansasii (NTM) : 12.3 copies/µL -
Component
2X qPCR Enzyme Mix
Oligo Mix (MTB/NTM)
Positive Control (MTB/NTM)
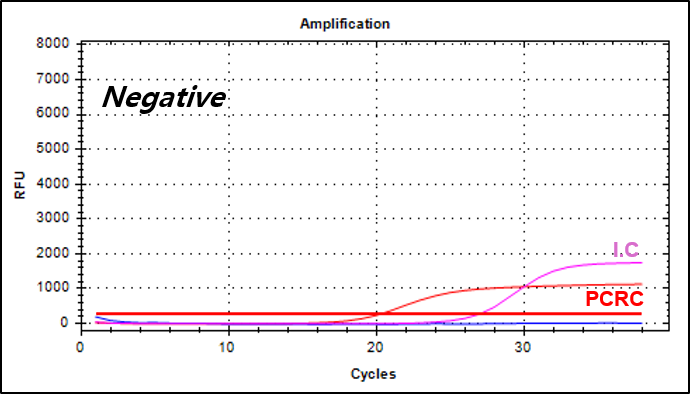
Negative Control -
Validated Instrument
CFX96 Dx system
Applied Biosystems 7500 Real-time PCR system
Applied Biosystems 7500 Fast Real-time PCR system -
Order No.
TB1001A (50 rxns / kit)
TB1001B (100 rxns / kit)
결과 그래프 (예시)