NextGene™ Influenza Virus Detection Kit (RUO)
- Simultaneous multiplex detection of major subtypes of Influenza A (H1N1 pdm09 and H3N2) and major subtypes of Influenza B (Victoria and Yamagata)
Avoiding the risk of contamination by applying UDG
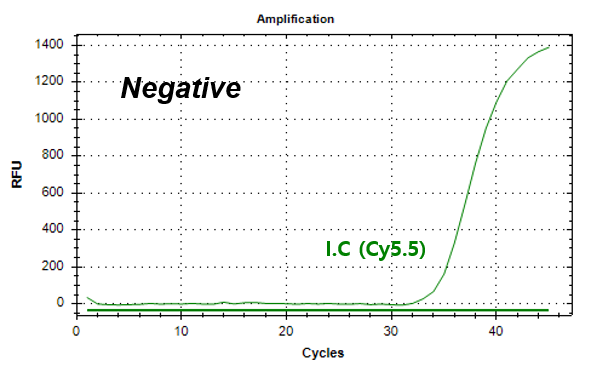
Monitoring whole PCR process by endogenous IC (Internal Control)
제품소개
MFDS export approval
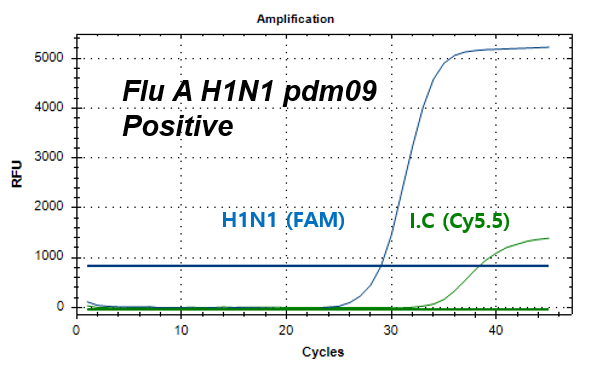
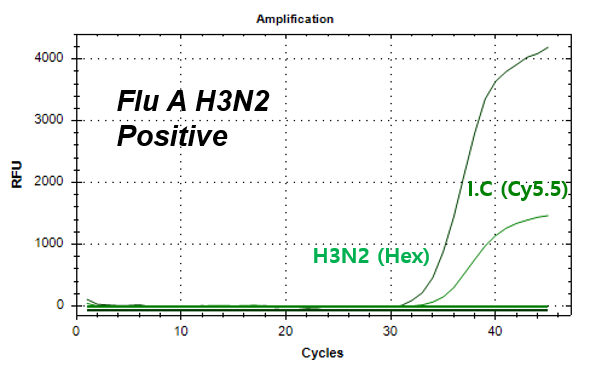
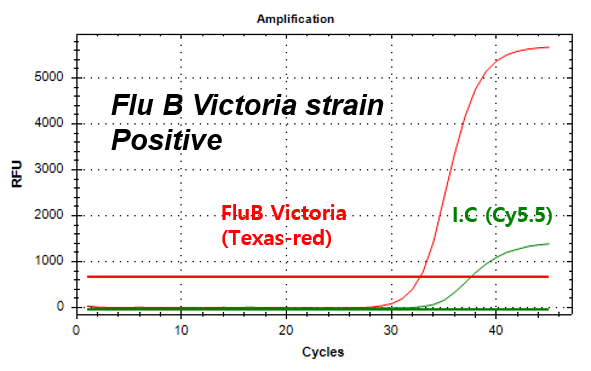
NextGene™ Influenza Virus Detection Kit is a real-time RT-PCR based reagent for research use, designed to simultaneously and qualitatively detect influenza A virus strains; A/H1N1 pdm09 and A/H3N2, and influenza B virus strains; B/Victoria and B/Yamagata from human nasopharyngeal swab or oropharyngeal swab samples.
제품정보
-
Detection Target
Influenza A-H1N1 pdm09
Influenza A-H3N2
Influenza B-Victoria
Influenza B-Yamagata -
Specimen
Nasopharyngeal swab, Oropharyngeal swab
-
Limit of Detection
Influenza A H1N1 pdm09 : 500 copies/mL
Influenza A H3N2 : 750 copies/mL
Influenza B Victoria : 500 copies/mL
Influenza B Yamagata : 750 copies/mL -
Component
2X RT-qPCR Enzyme Mix
Oligo Mix (Influenza virus)
Positive Control (Influenza virus)
Negative Control -
Validated Instrument
CFX96 Dx system
-
Order No.
RV1046A (50 rxns / kit)
RV1046B (100 rxns / kit)
결과 그래프 (예시)