NextGene™ HBV Quantification Kit (RUO)
- Quantitative detection to determine viral load
Avoiding the risk of contamination by applying UDG
Monitoring whole PCR process by endogenous IC (Internal Control)
제품소개
MFDS export approval
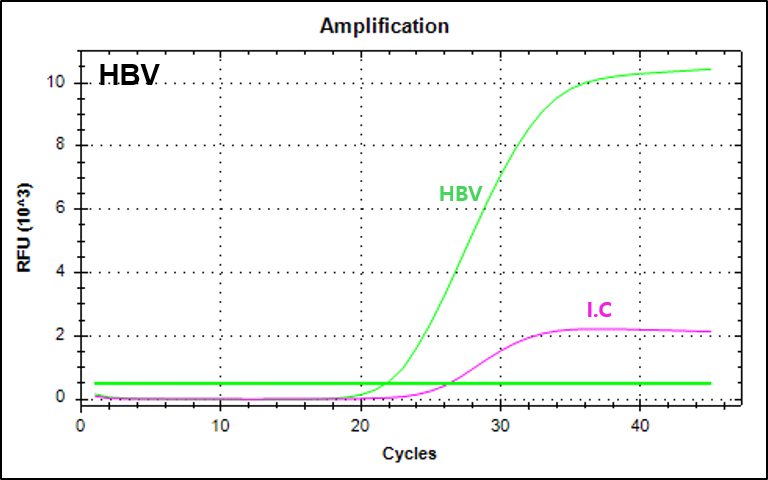
NextGene™ HBV Quantification Kit is a real-time PCR based reagent for research use, designed to quantitatively detect Hepatitis B virus from human serum or plasma samples. The viral load of hepatitis B can be measured with the kit.
제품정보
-
Detection Target
S gene of Hepatitis B virus
-
Specimen
Serum, Plasma
-
Limit of Detection
Serum : 84.5 IU/mL
Plasma : 84.5 IU/mL -
Component
2X qPCR Enzyme Mix
Oligo Mix (HBV)
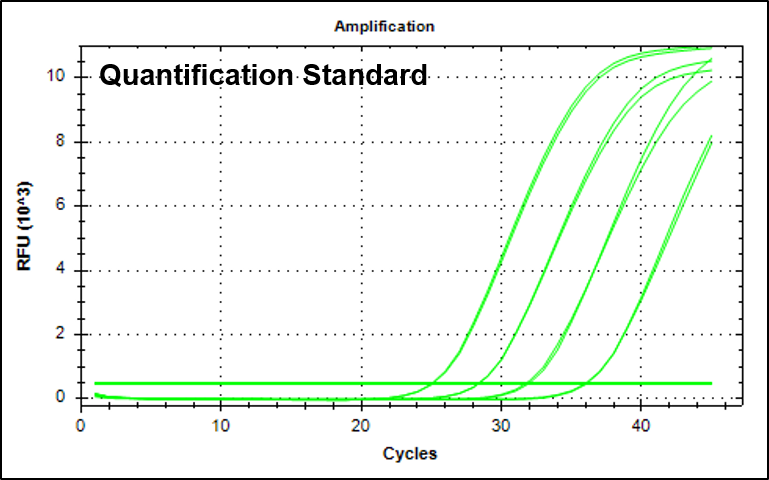
Quantification Standard A (HBV)
Quantification Standard B (HBV)
Quantification Standard C (HBV)
Quantification Standard D (HBV)
Negative Control -
Validated Instrument
CFX96 Dx system
-
Order No.
HB1001A (50 rxns / kit)
HB1001B (100 rxns / kit)
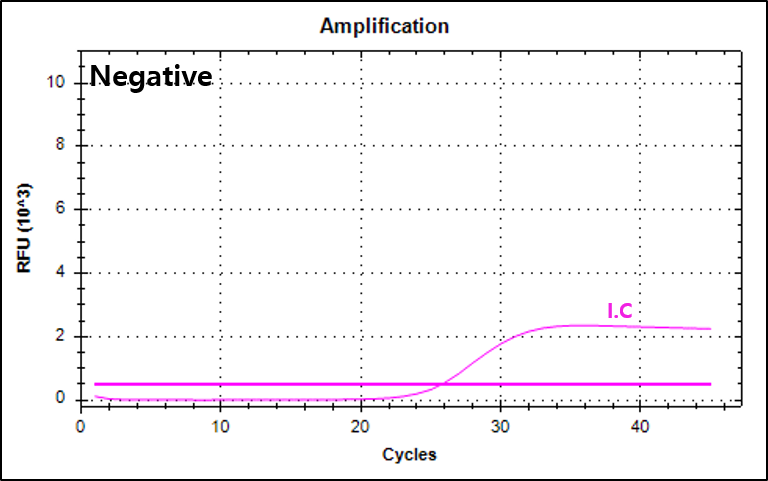
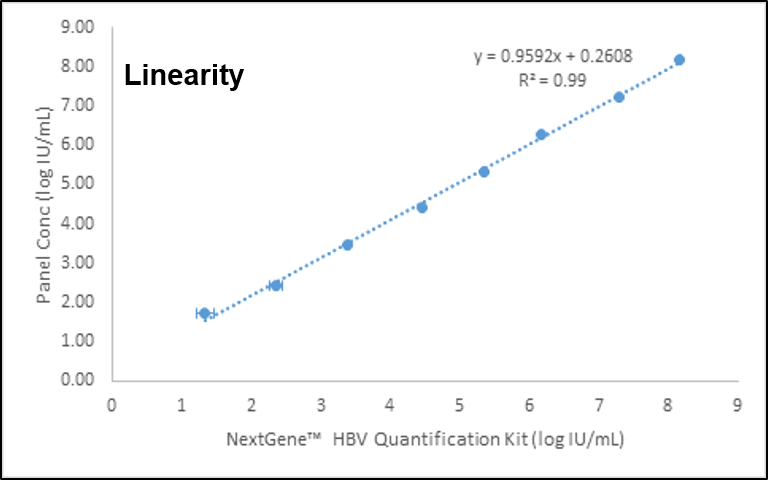
결과 그래프 (예시)