NextGene™ ApoE Genotyping Kit
- Simultaneous multiplex detection of six (6) genotype combinations associated with dementia risk genes in a single tube reaction
Avoiding the risk of contamination by applying UDG
제품소개
MFDS approval
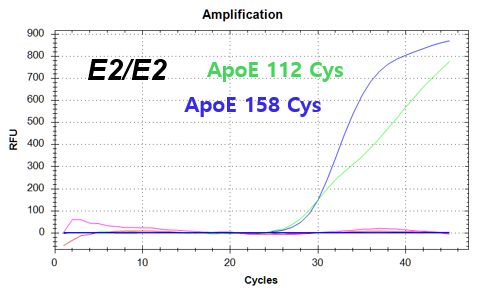
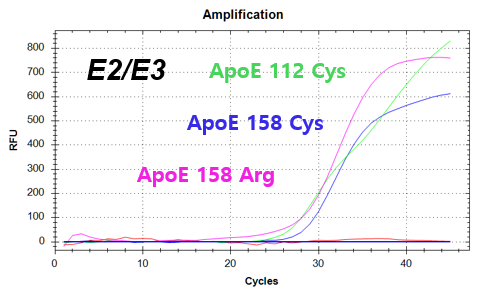
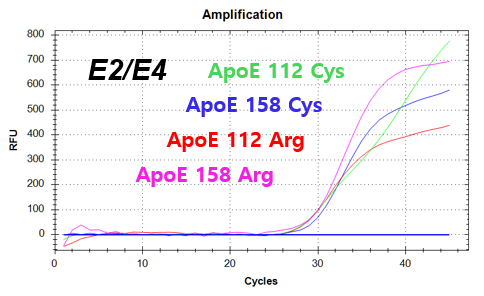
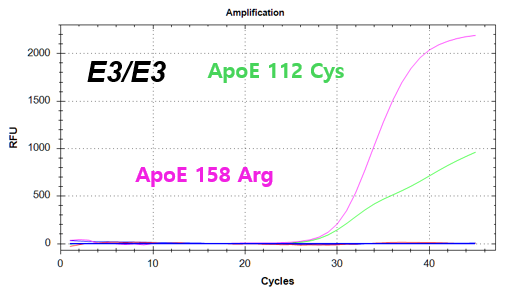
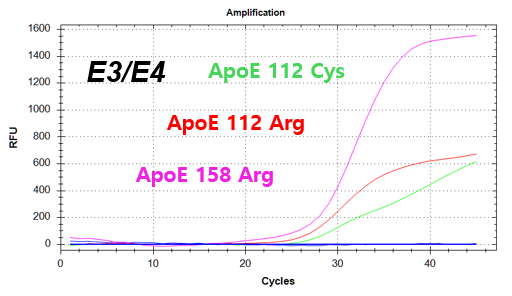
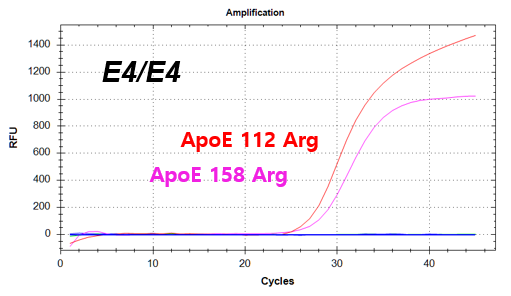
NextGene™ ApoE Genotyping Kit is a real-time PCR based in vitro diagnostic medical device designed to qualitatively detect dementia risk genes from human whole blood samples. The product identifies dementia risk human genotypes by detecting each combination of E2, E3, and E4 alleles of the Apolipoprotein E (ApoE) gene.
제품정보
-
Detection Target
Codon 112 (Cys/Arg) of the Apolipoprotein E (ApoE) gene
Codon 158 (Arg/Cys) of the Apolipoprotein E (ApoE) gene -
Specimen
EDTA whole blood
-
Limit of Detection
Genotype E2/E2 : 31.7 pg/µL
Genotype E2/E3 : 94.2 pg/µL
Genotype E2/E4 : 80.4 pg/µL
Genotype E3/E3 : 41.6 pg/µL
Genotype E3/E4 : 376.3 pg/µL
Genotype E4/E4 : 205.4 pg/µL -
Component
2X qPCR Enzyme Mix
Oligo Mix (ApoE)
Positive Control (ApoE)
Negative Control -
Validated Instrument
CFX96 Dx system
-
Order No.
AP1010A (50 rxns / kit)
AP1010B (100 rxns / kit)
결과 그래프 (예시)