NextGene™ P. jirovecii Detection Kit (RUO)
- Avoiding the risk of contamination by applying UDG
Monitoring whole PCR process by endogenous IC (Internal Control)
Product Summary
MFDS export approval
NextGene™ P. jirovecii Detection Kit is a real-time PCR based reagent for research use, designed to qualitatively detect Pneumocystis jirovecii gene relevant to Pneumocystis pneumonia (PCP) infection from human sputum or bronchoalveolar lavage fluid (BALF).
DETAIL
-
Detection Target
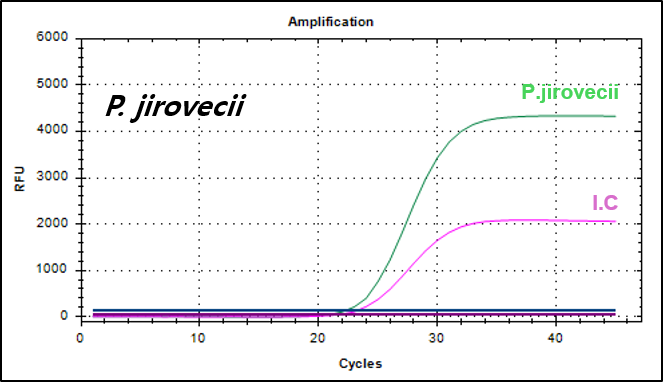
Pneumocystis jirovecii gene, the causative pathogen of Pneumocystis pneumonia (PCP)
-
Specimen
Sputum, Bronchoalveolar Lavage Fluid (BALF)
-
Limit of Detection
500 copies/mL
-
Component
2X qPCR Enzyme Mix
Oligo Mix (P. jirovecii)
Positive Control (P. jirovecii)
Negative Control -
Validated Instrument
CFX96 Dx system
-
Order No.
PJ1032A (50 rxns / kit)
PJ1032B (100 rxns / kit)
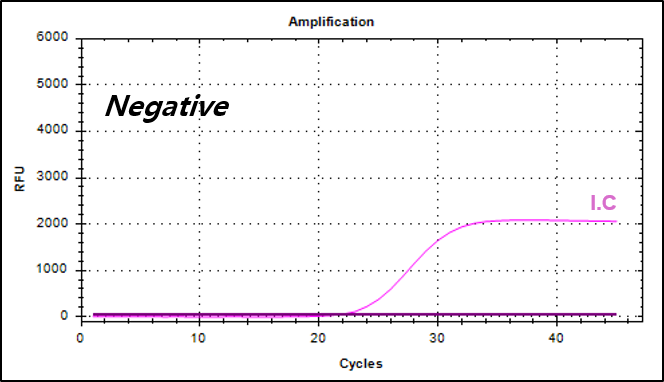
Example of Result