NextGene™ RP Bacteria Detection Kit (RUO)
- Simultaneous multiplex detection of three (3) different respiratory pneumonia causative pathogens
Avoiding the risk of contamination by applying UDG
Monitoring whole PCR process by endogenous IC (Internal Control)
Product Summary
MFDS export approval
NextGene™ RP Bacteria Detection Kit is a real-time PCR based reagent for research use, designed to simultaneously and qualitatively detect Bordetella pertussis, Streptococcus pyogenes, and Mycoplasma pnuemoniae from human sputum, nasopharyngeal swab, or oropharyngeal swab samples.
DETAIL
-
Detection Target
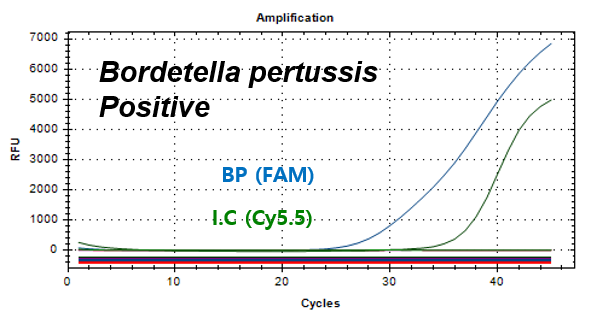
Bordetella pertussis
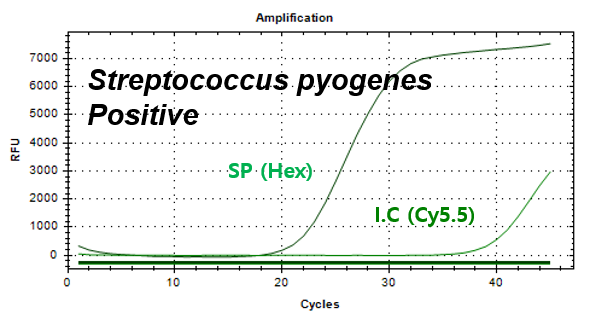
Streptococcus pyogenes
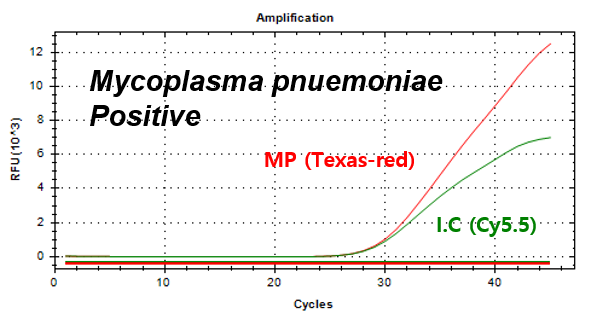
Mycoplasma pnuemoniae -
Specimen
Nasopharyngeal swab, Oropharyngeal swab, Sputum
-
Limit of Detection
Bordetella pertussis : 500 copies/mL
Streptococcus pyogenes : 500 copies/mL
Mycoplasma pneumoniae : 500 copies/mL -
Component
2X qPCR Enzyme Mix
Oligo Mix (RP Bacteria)
Positive Control (RP Bacteria)
Negative Control -
Validated Instrument
CFX96 Dx system
-
Order No.
RB1047A (50 rxns / kit)
RB1047B (100 rxns / kit)
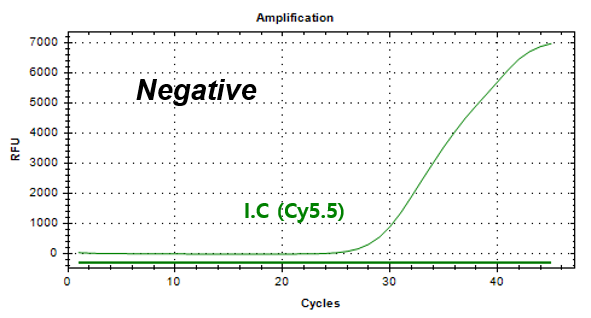
Example of Result